Better solubility and permeability
The active pharmaceutical ingredients can be divided into 4 classes. The active ingredients of the first class can be dissolved well and have good permeability. The active ingredients in the second class have good permeability but poor solubility. The active ingredients in the third class have good solubility but poor permeability. And finally, the active ingredients in the fourth class have poor solubility and also poor permeability.
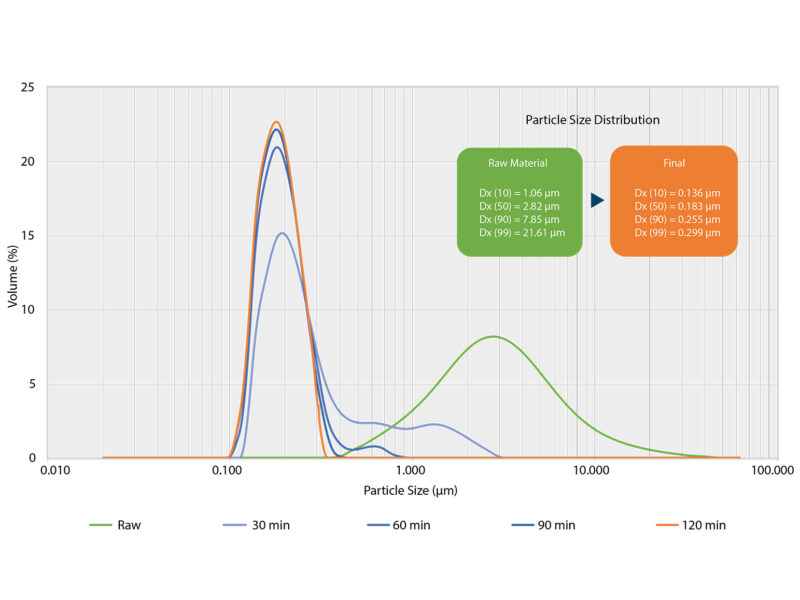
The particle size distribution of the active ingredients has a central influence on solubility and permeability. With smaller particle sizes, the surface area of the active ingredients is increased. This can increase efficiency, solubility and bioavailability. Active ingredients in the second class have good permeability per se, but are very difficult to dissolve. Wet milling involves the precise comminution and dispersion of these active ingredients to bring them into a well-soluble form.
Active pharmaceutical ingredients find wide applications in the pharmaceutical industry. They are used in various sectors, including the field of ophthalmic agents, injectables, auricular agents, oral applications, veterinary medicine, dermal applications and, last but not least, in the field of cell disruption for vaccine production.
The active pharmaceutical ingredients are mixed with a carrier liquid and then ground with a WAB-GROUP agitator bead mill. During this process, all parameters, such as maximum process temperature, must be maintained in order to achieve the desired particle properties.
It is important to note that processing of the active pharmaceutical ingredients must be carried out in strictly controlled environments and in compliance with all relevant regulatory requirements, as it is a critical step in drug manufacturing. WAB-GROUP has been providing the necessary process technologies to accomplish this key step in processing and the active pharmaceutical ingredients for over 50 years.
In the application areas of ophthalmic drugs, ear remedies and injectables, active pharmaceutical ingredients are produced in an aseptic environment. In order to comply with these criteria, agitator bead mills from WAB-GROUP are used, which meet the requirements of Cleaning in Place (CIP) as well as Sterilization in Place (SIP) and are qualified for these processes.
With the Cleaning in Place (CIP) process, we ensure that the operator of the plant does not come into contact with the active pharmaceutical ingredients. Complete cleaning of the agitator bead mill ensures that the hazards of the highly active pharmaceutical ingredients are always under control. Sterilization by means of steam heated up to 135° makes the plant completely sterile and enables germ-free production to be ensured. The necessary technical solutions have been developed and perfected over decades by WAB-GROUP engineers. Parameters such as sterilization, always safe cleaning, validation and qualification, monitoring of temperatures, requirements for technical components in handling heat, sterilization times, integration into existing automation chains, requirements electronic records and electronic signatures, seamless tracking of production, assurance of material quality, certification of product contact parts and much more, are an absolute standard of WAB-GROUP in connection with the processing of active pharmaceutical ingredients.
Are you interested in the production of active pharmaceutical ingredients and would like to learn more about our solutions on this topic? Then contact us via the contact form and talk to one of our process experts worldwide.

Contact Switzerland
Would you like information or advice on our products? Our team is at your disposal.

Always there for you
Our services
- Expert AdviceExpert Advice
- Spare PartsSpare parts
- RepairsRepairs
- TrialsTrials
- Trainings and SeminarsTrainings and seminars
WE ARE HERE FOR YOU
With our local sites and technical specialists on site, we are happy to be at your service as a competent engineering company and proven process expert.